우리 인류의 삶에 득이될까? 해가될까? 돼지 콩밭, 인간에 이식성공했다. 우선 이소식만 들었을때는 환영 또 대환영할 희소식임에는 틀림없다. 우리 인간의 얕삭한 상혼이 돼지콩팥을 인간에게 이식하는데 행여라도 Bait가 돼서는 안된다는 생각이 들었기 때문이다.
인류의 메디칼 역사상 처음으로 돼지의 콩팥을 인간에게 이식하여, 인간의 면역시스템에 아무런 부작용없이, 성공하여, 이결과데로라면, 현재 인간의 콩팥이식수술에 필요한 콩팥기증자를 기다리고 있는 수많은 환자들에게 희소식이 아닐수 없다 하는 빅뉴스라고 생각된다.
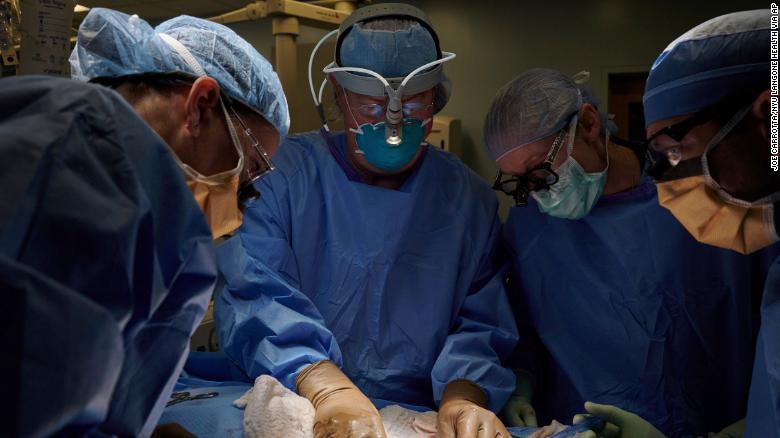
이번 수술은 뉴욕에 있는 NYU Langone Health에서 시행됐는데, 그냥 돼지 콩팥을 이식한게 아니고 이식하자마자 부작용발생을 미연에 방지하기위해 미리, 치명적일수있는 분자( molecule)가 들어있는 유전자 세포를 변형하여 이식을 했다고 한다. 이식받은 환자는 뇌사상태로 콩팥의 기능도 다 죽어가고 있었는데, 가족의 동의를 얻어, 생명연장 장치를 다 떼어내기전에 이식수술을 해본 것이다.
3일동안, 돼지콩팥은 여성환자의 몸밖에서혈관에 부착시키고, 지켜봤는데, 이를 연구하는 의료진들에게 긍정적인 반응이 나타난것이다. 이식된 콩팥은 극히 "정상적으로 아주 잘 작동되고있다"라고 이번 집도를 주관한 Dr. Robert Montgomery씨는 만족해 하고 있다.
닥터의 설명에 따르면 콩팥은 환자에 이식된 상태에서 예상이상으로 소변을 만들어 배출하고 있고, 돼지의 콩팥을 필요한 조치를 취하지 않고 다른 생명체에 이식했을때 금방 거부반응이 나타났었던 상황은 없었다고 자세히 설명해주고 있다.
이식수술후에 환자의 '크레아친' 수치는 극히 정상적이었고, 이수치는 콩팥의 작동이 원활치 않을때 나타나는 바로 미터인 셈이다 라고 Montgomery씨는 설명했다.
현재 미국에는 장기이식수술을 기다리는 환자의 수가 107,000이상이고, 그중에서 콩팥이식수술을 기다리는 환자는 90,000이라고, 장기 기증을 위해 수고하고 있는 United Network이 발표하고 있는데, 콩팥이식 수술을 기다리는 기간은 자그만치 3년에서 5년이라고 한다.
의료연구진들은 인간에게 장기이식수술을 하기위해 동물의 장기이식 가능성을 실험하는데 수십년씩을 연구해오고 있지만, 그러나 동물의 장기를 인간에게 이식수술을 했을때, 금방 발생하는 거부반응을 어떻게 방지해야 하는가에대한 진척 상황이 현재로서는 매우 어려운 상태인것으로 답보상태에 있다는 것이다.
이번 콩팥이식 수술은 단 3일간 지속됐었지만, 이번 수술로 앞으로의 수술에서 극복해야할 벽을 해소할수있는 서광이 조금은 보인것 같다고 Montgomery씨는 설명한다. 앞으로도 이러한 시험에 참가하게되는 환자는 인간의 콩팥을 이식받거나 투석하는데도 획복의 확률이 매우 적은 환자를 우선대상으로 하게 될것으로 보인다.
"암환자들이 회복될 가능성이 높다는 기대치를 갖고 있는것처럼, 암환자들에게 몇개월 더 생명연장 시키기위해 시도를 하거나 새로운 약을 개발하여 사용하는것에 대해 우리는 두번씩 생각을 하지는 않을 것이다."
Montgomery씨의 설명의 따르면, 뇌사상태의 환자들에게 시험수술을 하기위해 가족들과 충분한 상의를 하기전에, 의료연구진들은 지속적으로 '의료행위의 도덕성, 법과 종교적인 전문가들과 충분히 협의하고 일을 진행시킨다고 한다.
의료진들의 헌신적인 연구열에 찬사를 보내면서도, 법적 도덕적인 면에서 예상치 않은 장벽이 있을것 같다는 우려도 있다.
콩팥의 기능이 잘 작동돼지 않아 투석을 하거나 이식수술을 기다리는 환자가 놀랄 정도로 많다는것을 오늘 인식하면서, 나를 포함한 많은 건강 사람들은, 부모님에게 감사한 마음을 잊지 말아야겠다는 생각을 해보는게 어떨까?
The procedure done at NYU Langone Health in New York City involved the use of a pig whose genes had been altered so that its tissues no longer contained a molecule known to trigger almost immediate rejection.
The recipient was a brain-dead patient with signs of kidney dysfunction whose family consented to the experiment before she was due to be taken off life support, researchers told Reuters.
For three days, the new kidney was attached to her blood vessels and maintained outside her body, giving researchers access to it.
Test results of the transplanted kidney's function "looked pretty normal," said transplant surgeon Dr. Robert Montgomery, who led the study.
The kidney made "the amount of urine that you would expect" from a transplanted human kidney, he said, and there was no evidence of the vigorous, early rejection seen when unmodified pig kidneys are transplanted into non-human primates.
The recipient's abnormal creatinine level -- an indicator of poor kidney function -- returned to normal after the transplant, Montgomery said.
In the United States, nearly 107,000 people are presently waiting for organ transplants, including more than 90,000 awaiting a kidney, according to the United Network for Organ Sharing. Wait times for a kidney average three to five years.
Researchers have been working for decades on the possibility of using animal organs for transplants, but have been stymied over how to prevent immediate rejection by the human body.
Montgomery's team theorized that knocking out the pig gene for a carbohydrate that triggers rejection -- a sugar molecule, or glycan, called alpha-gal -- would prevent the problem.
The genetically altered pig, dubbed GalSafe, was developed by United Therapeutics Corp's Revivicor unit. It was approved by the US Food and Drug Administration (FDA) in December 2020, for use as food for people with a meat allergy and as a potential source of human therapeutics.
Medical products developed from the pigs would still require specific FDA approval before being used in humans, the agency said.
Other researchers are considering whether GalSafe pigs can be sources of everything from heart valves to skin grafts for human patients.
The NYU kidney transplant experiment should pave the way for trials in patients with end-stage kidney failure, possibly in the next year or two, said Montgomery, himself a heart transplant recipient. Those trials might test the approach as a short-term solution for critically ill patients until a human kidney becomes available, or as a permanent graft.
The current experiment involved a single transplant, and the kidney was left in place for only three days, so any future trials are likely to uncover new barriers that will need to be overcome, Montgomery said. Participants would probably be patients with low odds of receiving a human kidney and a poor prognosis on dialysis.
"For a lot of those people, the mortality rate is as high as it is for some cancers, and we don't think twice about using new drugs and doing new trials (in cancer patients) when it might give them a couple of months more of life," Montgomery said.
The researchers worked with medical ethicists, legal and religious experts to vet the concept before asking a family for temporary access to a brain-dead patient, Montgomery said.
The procedure done at NYU Langone Health in New York City involved the use of a pig whose genes had been altered so that its tissues no longer contained a molecule known to trigger almost immediate rejection.
The recipient was a brain-dead patient with signs of kidney dysfunction whose family consented to the experiment before she was due to be taken off life support, researchers told Reuters.
For three days, the new kidney was attached to her blood vessels and maintained outside her body, giving researchers access to it.
Test results of the transplanted kidney's function "looked pretty normal," said transplant surgeon Dr. Robert Montgomery, who led the study.
The kidney made "the amount of urine that you would expect" from a transplanted human kidney, he said, and there was no evidence of the vigorous, early rejection seen when unmodified pig kidneys are transplanted into non-human primates.
The recipient's abnormal creatinine level -- an indicator of poor kidney function -- returned to normal after the transplant, Montgomery said.
In the United States, nearly 107,000 people are presently waiting for organ transplants, including more than 90,000 awaiting a kidney, according to the United Network for Organ Sharing. Wait times for a kidney average three to five years.
Researchers have been working for decades on the possibility of using animal organs for transplants, but have been stymied over how to prevent immediate rejection by the human body.
Montgomery's team theorized that knocking out the pig gene for a carbohydrate that triggers rejection -- a sugar molecule, or glycan, called alpha-gal -- would prevent the problem.
The genetically altered pig, dubbed GalSafe, was developed by United Therapeutics Corp's Revivicor unit. It was approved by the US Food and Drug Administration (FDA) in December 2020, for use as food for people with a meat allergy and as a potential source of human therapeutics.
Medical products developed from the pigs would still require specific FDA approval before being used in humans, the agency said.
Other researchers are considering whether GalSafe pigs can be sources of everything from heart valves to skin grafts for human patients.
The NYU kidney transplant experiment should pave the way for trials in patients with end-stage kidney failure, possibly in the next year or two, said Montgomery, himself a heart transplant recipient. Those trials might test the approach as a short-term solution for critically ill patients until a human kidney becomes available, or as a permanent graft.
The current experiment involved a single transplant, and the kidney was left in place for only three days, so any future trials are likely to uncover new barriers that will need to be overcome, Montgomery said. Participants would probably be patients with low odds of receiving a human kidney and a poor prognosis on dialysis.
"For a lot of those people, the mortality rate is as high as it is for some cancers, and we don't think twice about using new drugs and doing new trials (in cancer patients) when it might give them a couple of months more of life," Montgomery said.
The researchers worked with medical ethicists, legal and religious experts to vet the concept before asking a family for temporary access to a brain-dead patient, Montgomery said.
https://www.cnn.com/2021/10/20/health/human-pig-kidney-transplant-scli-intl-scn/index.html



No comments:
Post a Comment